by Steve Donnigan | Jan 28, 2026 | Uncategorized
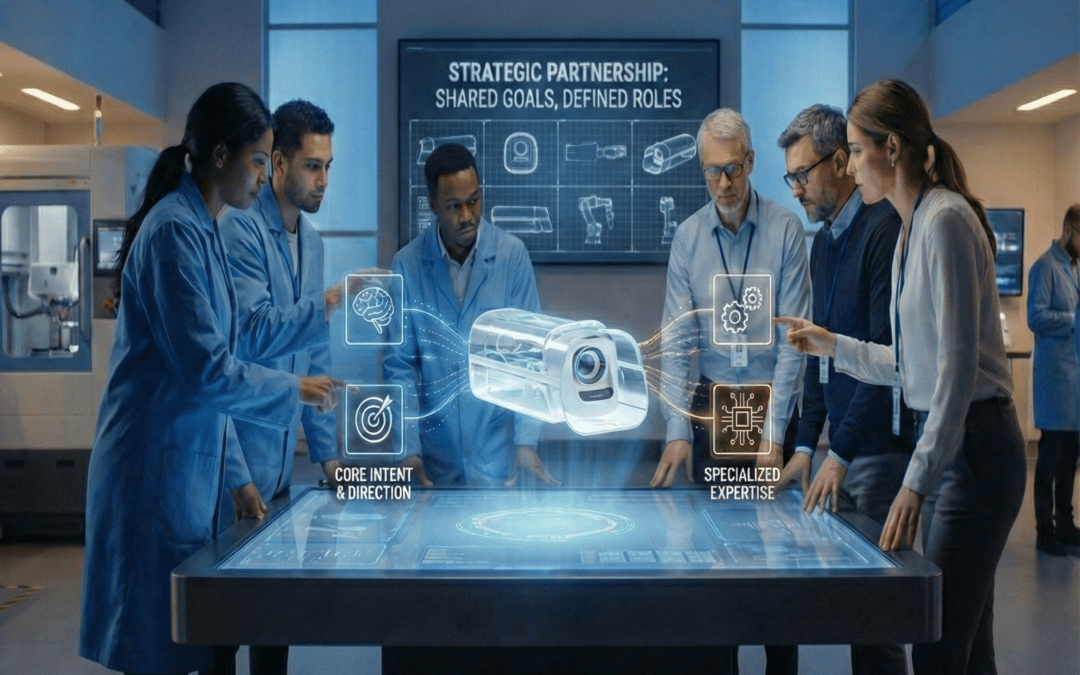
Medical device organizations today are operating in a tighter, more complex environment than ever before. Regulatory scrutiny continues to rise, technology stacks are converging, development timelines are compressing, and cost pressure is unrelenting. In this context,...

by Steve Donnigan | Jan 21, 2026 | Uncategorized
Outsourcing Engineering Is a Strategic Business Decision Medical device startups rarely fail because of insufficient engineering effort, they fail because scarce resources such as capital, time, and attention are misallocated. Outsourcing engineering is often...

by Steve Donnigan | Dec 12, 2025 | Uncategorized
How to Get Them Right Designing a successful medical device is a disciplined process rooted in systemized product development and advanced regulatory compliance, not luck or guesswork. Every life saving product on the market today has passed through FDA-mandated...