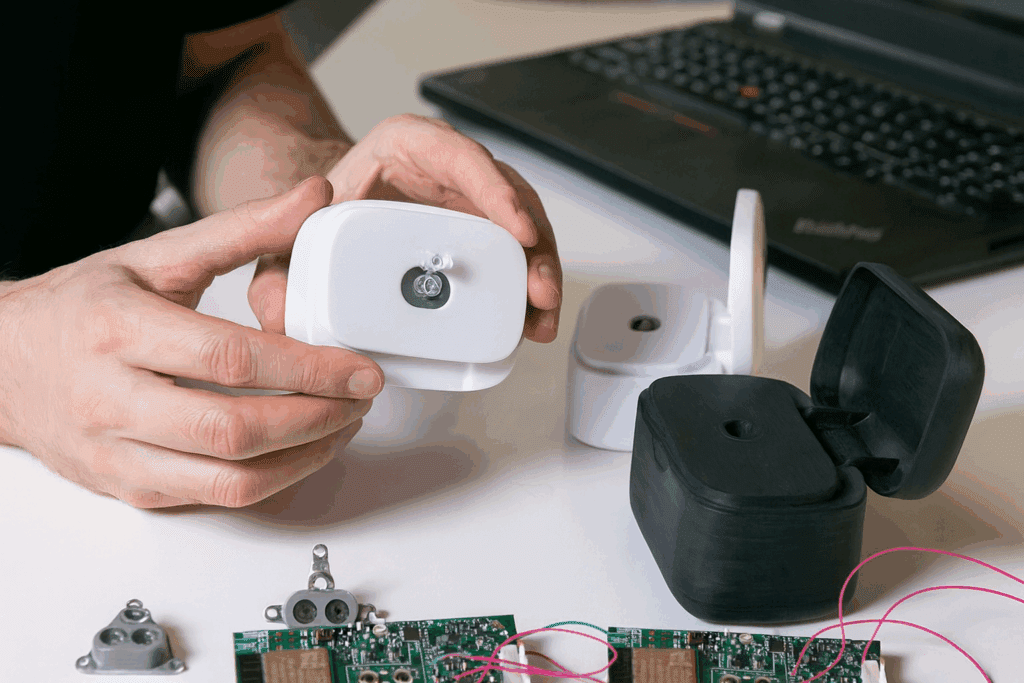
Medical device prototyping is a specialized process that helps turn ideas into working products for the healthcare industry. This process involves building models and early versions of devices, allowing teams to see how designs work in real life before the final product is made.
Prototyping medical devices requires careful planning, precise execution, and attention to strict rules set by regulators. Unlike other types of product prototyping, medical devices often demand additional documentation, risk analysis, and testing because they are used in healthcare settings.
This article explores the main stages and best practices for medical device prototyping. Each section explains what makes this process unique and offers clear steps to guide successful development.
Understanding Medical Device Prototyping
Medical device prototyping is the process of creating early models or samples of a medical device to test design ideas, functions, and usability during development. These models help development teams check performance, safety, and user needs before making the final version for clinical use.
There are different types of medical prototypes:
- Proof-of-concept prototypes: Show that a core idea can work
- Functional prototypes: Include working features and let teams test specific components or systems
- Pre-production prototypes: Nearly identical to the final product and used for final testing and regulatory review
Prototyping medical devices involves unique challenges compared to general product prototyping. Development teams manage strict regulatory requirements, such as FDA or EU MDR guidelines. About 75% of US-based medical device startups fail, with failures attributed to financial burdens, regulatory hurdles, and technical challenges in development. Documentation, traceability, and risk management are essential throughout the process.
Key terms include design input (requirements from users or regulations), design output (what the prototype delivers), verification (testing if the device meets requirements), and validation (confirming the device meets user needs). Medical device prototyping connects technical work with regulatory pathways, aiming to ensure safety and effectiveness for patients and users.
Why Early Prototyping Matters
Starting the medical device prototype process early in development allows teams to find and address design issues before they become more difficult and expensive to fix. Early detection of problems with safety, performance, or usability can prevent delays in the project timeline and helps avoid expensive changes that may be required if the device design is already advanced.
Introducing prototypes early in development also supports clear communication with stakeholders such as investors, clinicians, and regulatory reviewers. Seeing and interacting with an early model builds understanding and trust, making it easier to get feedback and support throughout the project.
Common Pitfalls In Prototyping Medical Devices
Prototyping medical devices is a complex process with common errors that can affect a project’s timeline, safety, and cost:
- Skipping user input: Developing prototypes without involving clinicians or end-users can result in devices that are hard to use or do not meet real clinical needs
- Inadequate documentation: Failing to record design decisions, test results, and changes makes it difficult to track what has been done and to meet regulatory requirements
- Overlooking regulatory requirements: Not aligning the prototype process with regulatory standards may cause rework if prototypes do not meet safety or performance criteria
- Poor risk management: Ignoring risk assessment during prototyping medical devices can lead to undetected hazards
Warning signs of potential prototyping problems include repeated test failures, confusion about design decisions, missing or incomplete records, and feedback from users or reviewers that is not addressed.
The Step-By-Step Framework For Successful Medical Device Prototyping
1. Define Requirements And Objectives
Medical device prototyping begins with clear goals for each prototype. Teams collect input from clinicians and users to understand what the device needs to do in real-world settings. These clinical needs are translated into technical requirements, such as performance standards, size, and safety criteria.
Requirements are often prioritized based on risk, user impact, and regulatory demands. Documentation is created to keep track of all inputs, specifications, and acceptance criteria.
2. Develop Initial Concept Designs
Medical prototype design moves from written requirements to visual concepts. Teams use brainstorming, sketching, and 3D modeling to generate multiple design ideas. Each concept is evaluated for technical feasibility, user needs, and potential for regulatory approval.
Selection criteria include how well designs meet requirements, how easy they are to manufacture, and anticipated user experience. Sketches, renderings, and basic physical models help teams compare and refine concepts before choosing those to advance.
3. Select Materials And Manufacturing Methods
Material selection for prototypes depends on the device’s function, the stage of prototyping, and regulatory expectations. Teams compare materials based on properties such as biocompatibility, strength, and sterilization compatibility.
Common materials include:
- ABS Plastic: Low cost, suitable for early prototypes, limited sterilization options
- Polycarbonate: High biocompatibility, good sterilization tolerance, medium cost
- Stainless Steel: Excellent sterilization, high biocompatibility, higher cost for implantable components
- Silicone: High biocompatibility, good sterilization, medium cost for soft tissue interfaces
Methods such as 3D printing, CNC machining, and injection molding are selected based on prototype stage. Teams balance level of detail with available resources and timelines.
4. Build Functional Prototypes
Prototypes are constructed to test how the device works. Complex devices may be divided into subsystems for separate testing. Quality control measures are used during assembly, such as inspecting parts and documenting each step.
Records of materials, assembly steps, and any deviations are maintained for traceability. Each medical prototype is built to check specific functions, such as electrical safety, mechanical movement, or ease of cleaning.
5. Conduct Testing And Validation
Testing verifies that prototypes meet requirements and perform as intended. Teams select test types based on the prototype’s stage, such as mechanical testing, electrical safety, or usability trials. Test protocols are developed before testing begins, outlining steps, equipment, and criteria for passing.
When tests fail, results are reviewed to determine if the issue is with the design, materials, or assembly. Analysis guides changes for the next round.
6. Gather User Feedback
User feedback is collected from clinicians, patients, or caregivers who interact with the prototype. Structured feedback sessions may use interviews, surveys, or observation during simulated use.
Feedback is analyzed systematically, looking for patterns and areas where users struggle or suggest changes. Conflicting opinions are considered by weighing clinical importance and technical feasibility.
7. Refine And Iterate
Results from testing and user feedback inform the next iteration of the prototype. Changes are prioritized based on safety, performance, and regulatory impact. All revisions are tracked in design records to maintain a clear history.
Decisions are made whether to adjust the design, explore a new direction, or finalize the prototype. Prototyping concludes when design goals, user needs, and regulatory criteria are all satisfied.
Managing Risk, Testing, And Regulatory Requirements
Risk management is an ongoing part of medical device prototyping. Each prototype version provides information about possible hazards or failures. Teams use this information to create and update risk analysis documents, such as Failure Modes and Effects Analysis (FMEA), to show how risks are identified, controlled, and monitored during development.
Regulatory agencies expect detailed records from the medical device prototyping process. Documentation includes design inputs and outputs, change histories, test plans, and results. These documents support regulatory submissions by providing evidence that the device is being developed according to standards.
Testing protocols during prototyping align with regulatory standards like ISO 13485 or FDA guidance. Protocols describe what tests will be performed, how they are conducted, what equipment is used, and what results are expected.
How To Incorporate Clinical Feedback And User Testing
Clinical feedback and user testing are important steps for developing medical prototypes that perform well in real life. Structured sessions with healthcare professionals and end-users often use interviews, focus groups, or observation while the device is being used.
Simulating real-life use environments can involve setting up a mock hospital room, operating room, or patient care area where users interact with the prototype as they would in daily practice. Controlled simulations help identify issues with device handling, workflow, or safety that might not appear in a regular lab setting.
Several 3D printing technologies are commonly used in medical device prototyping: the global healthcare additive manufacturing market was valued at $7.4 billion in 2022 and is expected to grow at a compound annual growth rate of 18.1% from 2023 to 2030.
Key Materials And Methods For Medical Prototypes
3D Printing Techniques
Several 3D printing technologies are commonly used in medical device prototyping:
- Fused Deposition Modeling (FDM): Prints objects layer by layer using thermoplastic filaments, suitable for quick, low-cost prototypes but with limited surface detail
- Stereolithography (SLA): Uses liquid resin cured by a laser to create highly detailed models with smooth surfaces, offering more precision for parts requiring fine features
- Selective Laser Sintering (SLS): Fuses powdered materials like nylon using a laser, producing strong, functional parts with complex geometries
3D printing enables rapid iteration and low-volume production. However, some 3D-printed materials may not be suitable for sterilization or long-term contact with the body.
CNC Machining
CNC (Computer Numerical Control) machining uses computer-guided tools to precisely cut or shape materials such as metals and plastics. This method is often used for prototypes that require tight tolerances, complex geometries, or high structural integrity.
Common materials for CNC machining include aluminum, stainless steel, titanium, polycarbonate, and PEEK. The method is more expensive and time-consuming than 3D printing for complex shapes but provides parts suitable for rigorous testing.
Injection Molding
A65 Consulting approaches medical device prototyping by working closely with clients from the earliest design ideas through to market-ready products. This process involves clear communication, technical planning, and careful management of documentation to align with industry standards. Some companies are investing more than five hours per week in managing MDR compliance, with costs potentially consuming 6% to 10% of their annual revenues.
The upfront cost of creating a mold is high, but the cost per part decreases as more parts are made. Injection molding is used to produce high-fidelity prototypes that closely match the appearance, function, and material properties of final products.
Partner With A65 Consulting For End-To-End Prototyping Solutions
A65 Consulting approaches medical device prototyping by working closely with clients from the earliest design ideas through to market-ready products. This process involves clear communication, technical planning, and careful management of documentation to align with industry standards.
Medical device prototyping typically accounts for 15-25% of overall development costs, but early prototyping can reduce total project expenses by identifying design problems and regulatory challenges sooner in the process. Clinical trials for medical device FDA approval range from $1 million to $10 million depending on complexity, while preclinical testing costs range from $10,000 to $500,000.
Medical device prototyping typically accounts for 15-25% of overall development costs, but early prototyping can reduce total project expenses by identifying design problems and regulatory challenges sooner in the process. The rapid prototyping medical devices market is projected to reach $7.78 billion by 2033, with a compound annual growth rate of 15% from 2025 to 2033.
Frequently Asked Questions About Medical Device Prototyping Success
How much does medical device prototyping typically cost compared to total development expenses?
Medical device prototyping typically accounts for 15-25% of overall development costs, but early prototyping can reduce total project expenses by identifying design problems and regulatory challenges sooner in the process.
What timeline do companies typically experience for comprehensive medical device prototyping?
The timeline for medical device prototyping depends on the complexity of the device. Simple prototypes often take 2-4 months, while more complex systems can require 6-12 months for complete iteration.
How do developers handle electronic and software integration in medical device prototypes?
A modular approach separates hardware and software components in the early stages, allowing parallel development and testing. Commercial off-the-shelf components often validate the core function before integrating custom solutions.
How do companies choose external prototyping partners when lacking in-house resources?
Selection of an external partner often involves reviewing their experience with medical devices, understanding of regulatory requirements, established quality systems, and documentation practices that support regulatory submissions.

